Oct 15, 2015
Introduction
By Stephen Leong, MD
University of Colorado Cancer Center
There have been significant advances in the treatment of metastatic colorectal cancer. New chemotherapy has improved survival and response rates. Advances in surgical technique and expanded criteria for resectability of liver metastases have further improved survival rates.1-4
However, 75% to 90% of patients will still have unresectable metastases.5,6 For these patients, there are a number of liver-directed therapy options: radiofrequency ablation, microwave ablation, cryoablation, hepatic artery infusion (HAI), chemoembolization, radioembolization, and stereotactic body radiation therapy (SBRT).
There is growing evidence to support the use of these modalities, but there is a lack of large randomized trials comparing them to one another.7-13 There is also the question about the relative advantages of different endpoints: Should we focus on rate of conversion from unresectability to resectability, response rate, or disease control rates? Regardless of the answer, liver-directed therapies have fully established themselves as part of the metastatic colorectal cancer armory.
In this article, two leading experts, Nancy E. Kemeny, MD, FASCO, a medical oncologist at Memorial Sloan Kettering Cancer Center, who is a pioneer in HAI, and Sarah E. Hoffe, MD, Section Head of Gastrointestinal Radiation Oncology at Moffitt Cancer Center, who has expertise in advanced radiation therapy techniques including SBRT, debate the current role and benefits of these treatment options.
Dr. Leong is an Associate Professor in the Division of Medical Oncology at the University Of Colorado School of Medicine. An ASCO member since 2005, he is a member of the ASCO Connection Editorial Board.
References
- Cremolini C, Schirripa M, Antoniotti C, et al. Nat Rev Clin Oncol. Epub 2015 Jul 28.
- Choti MA, Sitzmann JV, Tiburi MF, et al. Ann Surg. 2002;235:759-66.
- Scheele J, Stang R, Altendorf-Hofmann A, et al. World J Surg. 1995;19:59-71.
- Pedersen IK, Burcharth F, Roikjaer O, et al. Dis Colon Rectum. 1994;37:1078-82.
- Morris EJ, Forman D, Thomas JD, et al. Br J Surg. 2010;97:1110-8.
- Manfredi S, Lepage C, Hatem C, et al. Ann Surg. 2006;244:254-9.
- Choti MA. Curr Treat Options Oncol. 2014; 15:456-64.
- Raval M, Bande D, Pillai AK, et al. Front Oncol. 2014;4:120.
- Zurkiya O, Ganguli S. Front Oncol. 2014; 4:150.
- Timmerman RD, Bizekis CS, Pass HI, et al. CA Cancer J Clin. 2009;59:145-70.
- Garrean S, Hering J, Saied A, et al. Am J Surg. 2008;195:508-20.
- Qiu H, Katz AW, Chowdhry AK, et al. Am J Clin Oncol. Epub 2015 Aug 11.
- Petre EN, Sofocleous CT, Solomon SB. Hematol Oncol Clin North Am. 2015;29:117-33.
The Rationale for HAI
By Nancy E. Kemeny, MD, FASCO
Memorial Sloan Kettering Cancer Center
Improvements in the treatment of metastatic colorectal cancer in the last few decades have increased response rates (RRs) and survival, but unfortunately, in most trials, 50% of patients are not surviving the disease after 30 months. Effective new treatments need to be developed. The rationale for HAI are: 1) hepatic metastases are nourished by the hepatic artery, while normal hepatocytes are fed by the portal vein; 2) there may be a stepwise pattern of metastatic progression, first to the liver and then to the lung and to other organs; and 3) agents that are taken up by the liver via the first-pass effect produce a significant concentration of drugs in the tumor while minimizing systemic toxicity.1,2 The ideal agent for HAI is floxuridine, with a reported 94% to 99% extraction rate by the liver during the first-pass—a 10-fold higher rate than that obtained with 5-fluorouracil (FU).3
A number of randomized trials comparing HAI therapy to FU-based systemic therapy demonstrate a significant increase in RR and progression-free survival with HAI chemotherapy in patients with hepatic-only colorectal metastases. In these studies, information on overall survival (OS) was hampered by a small number of patients in some trials or crossover from systemic to HAI therapy after progression on systemic therapy. A CALGB study of HAI therapy versus systemic FU/leucovorin (LV) in 135 patients without crossover revealed an increase in survival of 24.4 months versus 20 months and an RR of 47% and 24% for HAI versus systemic therapy, respectively.4
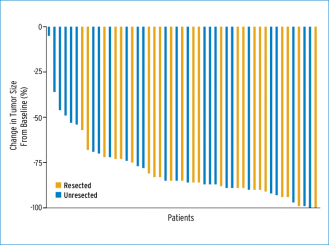
Although no phase III trials involving HAI in combination with systemic therapy have been conducted, several groups have examined the safety and efficacy of combination therapy in phase I and II trials. Memorial Sloan Kettering Cancer Center (MSKCC) carried out a phase I study in which 46 previously treated patients received HAI and systemic irinotecan; patients had an RR of 74% and a 20-month median survival from the time of HAI initiation.5 A phase I trial of systemic oxaliplatin and irinotecan combined with HAI floxuridine/dexamethasone produced an RR of 88% in 36 patients (89% previously treated), with a median survival of 36 months.6 A phase II study carried out by Ducreux et al. found that patients who received HAI oxaliplatin and systemic 5-FU/LV reported an RR of 64% and an OS of 27 months.7 A newer study using HAI oxaliplatin, irinotecan, and FU as second-, third-, or fourth-line therapy found a median survival of 31 months and that R0 to R1 resections were performed on 45% of patients who were treated in the second-line setting.8 Another phase I trial at MSKCC gave HAI plus systemic oxaliplatin and irinotecan to patients with unresectable liver metastases and found a complete or partial response rate of 92%; the waterfall curve in that study showed that 75% of these patients had a greater than 75% reduction in disease (Fig. 1).9
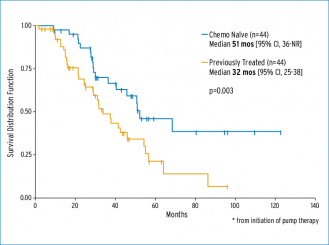
One strategy is to convert patients with unresectable liver disease to resection. As shown in Fig. 1, the quality of the response to HAI therapy allows more patients to get to resection. A prospective trial of HAI plus systemic chemotherapy evaluated patients with unresectable disease, defined as margin-negative resection requiring resection of three hepatic veins, portal veins, or the retrohepatic vena cava, or a resection that leaves fewer than two adequately perfused and drained segments; the trial found that 47% of patients were converted to resection.10 In a review of 105 patients from two trials using HAI and systemic therapy, conversion to resection was possible in 44% of previously treated patients and in 57% of chemotherapy-naive patients.11 Survival curves for these patients are shown in Fig. 2.
In conclusion, RRs have been shown to be higher with HAI, especially when used with systemic therapy, allowing for better systemic control while continuing to provide the liver with high concentrations of the active drug to maintain hepatic control. Combined therapy can prolong the survival of patients with advanced colorectal cancer and allow resection in almost one-half of the patients.
Dr. Kemeny is a medical oncologist and clinical researcher at Memorial Sloan Kettering Cancer Center. An ASCO member since 1988, she has served on the Board of Directors, as Chair of the Membership Committee and the Special Awards Selection Committee, and as a member of the Gastrointestinal Tumor Markers Expert Panel. Dr. Kemeny received the distinction of Fellow of ASCO (FASCO) in 2007.
References
- Breedis C, Young C. Am J Pathol. 1954; 30:969-77.
- Weiss L, Grundmann E, Torhorst J, et al. J Pathol. 1986;150:195-203.
- Ensminger WD, Gyves JW. Semin Oncol. 1983;10:176-82.
- Kemeny NE, Niedzwiecki D, Hollis DR, et al. J Clin Oncol. 2006;24:1395-403.
- Kemeny N, Gonen M, Sullivan D, et al. J Clin Oncol. 2001;19:2687-95.
- Kemeny N, Jarnagin W, Paty P, et al. J Clin Oncol. 2005;23:4888-96.
- Ducreux M, Ychou M, Laplanche A, et al. J Clin Oncol. 2005;23:4881-7.
- Ducreux M, Innominato PF, Hebbar M, et al. J Clin Oncol. 2013;31 (suppl; abstr 3599).
- Kemeny NE, Melendez FD, Capanu M, et al. J Clin Oncol. 2009;27:3465-3471.
- D'Angelica MI, Correa-Gallego C, Paty PB, et al. Ann Surg. 2015;261:353-60.
- Kemeny NE, Fong Y, Paty P, et al. J Clin Oncol. 2012;30 (suppl; abstr 3577).
SBRT: An Effective Strategy
By Sarah E. Hoffe, MD
Moffitt Cancer Center
Since the mid-1990s, radiation oncologists have been exploring the potential of SBRT to deliver extremely potent ablative doses to moving extracranial tumor targets while ensuring steep gradients to avoid normal tissue injury.1 Multiple techniques have evolved since that time to enable the delivery of highly focused treatments, with doses ranging up to more than 10 times the conventional standard of 1.8 to 2.0 Gy per fraction, in one to five fractions.2 A recent survey suggests that SBRT has become a standard tool for physicians both in academic and community practices, with the majority having adopted this technology by 2008.3
There are radiobiologic concerns about the potential of SBRT to increase late effects to the surrounding normal tissue due to the high dose per fraction.4 These concerns are lessened, however, due to the precision of the SBRT technique.5 The ideal patient with liver-only metastases who is offered SBRT would have small targets with three or fewer lesions measuring 6 cm or less, with locations of at least a 1.5-cm distance from a luminal organ of the gastrointestinal tract.6
Evidence to date suggests that SBRT is an effective strategy for patients with unresectable colorectal liver metastases (uCRLM). Although no phase III or long-term survival data exists, multiple investigators have reported on recent phase I/II trials in patients with limited hepatic metastases from multiple primary sites. These studies found no significant toxicities associated with doses delivered in one to three to five fractions, with overall two-year local control rates of over 90%, as well as a more individualized dosing approach.7-10
When compared with other histologies, uCRLM may be more radioresistant and colorectal metastases in the liver may be more resistant to SBRT than those in the lung.11,12 Indeed, the total dose, dose per fraction, and the biologically equivalent dose (BED) have all been correlated with local control.13 A recent phase II trial, evaluating only patients with uCRLM, delivered a higher dose of 75 Gy in 3 fractions to 42 patients with a median tumor size of 3.5 cm and reported a two-year actuarial local control rate of 91%.14
With the incorporation of modern systemic chemotherapy regimens, response rates have increased, with up to 30% of patients experiencing a response sufficient for consideration of resection and possible cure.15 Yet, for the surgeon, technical issues can pose significant concerns, such as the proximity of the disease to major blood vessels or the possibility that the intended amount of liver to be removed may not leave enough future liver remnant (FLR) for function.16,17 In addition, there is the consideration to optimize the number of chemotherapy cycles to downstage the tumor while decreasing the risk of chemotherapy-associated steatohepatitis.18
The future question, then, is: how best to consider integration of SBRT into the potentially curative treatment paradigm for uCRLM? Surgical resection series have defined 10-year survival as the curative hallmark, since 34% of five-year survivors can still die of their disease. In a large series of 612 consecutive patients, 102 were 10-year survivors; of these, 7% had synchronous disease, 36% had a disease-free interval shorter than 12 months, 25% had bilobar metastases, 50% had a nodepositive primary, 39% had more than one metastasis, and 35% had a tumor size larger than 5 cm.19 These numbers indicate that poor prognostic factors, such as those based on the clinical risk score, do not preclude cure, except in the setting of positive margins.20
Since SBRT is effective for multiple small tumors, its future role for conversion patients might lie in enhancing margin-negative resection for those lesions the surgeon deems at risk for a positive margin. Further studies are needed to ensure that the transient decline in normal liver volume seen within the first three to six months after SBRT and before regeneration occurs would not preclude the viability of the FLR.
Dr. Hoffe is the Section Head of Gastrointestinal Radiation Oncology and an Associate Member of the faculty at Moffitt Cancer Center. She is also an Associate Professor in the Department of Oncologic Sciences at the University Of South Florida Morsani College of Medicine.
References
- Papiez L, Timmerman R, DesRosiers C, et al. Acta Oncol. 2003;42:882-94.
- Timmerman RD, Kavanagh BD, Cho LC, et al. J Clin Oncol. 2007;25:947-52.
- Pan H, Simpson DR, Mell LK, et al. Cancer. 2011;117:4566-72.
- Fletcher GH. Radiother Oncol. 1991;20:10-5.
- Rosenthal DI, Glatstein E. Oncologist. 1996;1:1-7.
- Schefter TE, Kavanagh BD. Semin Radiat Oncol. 2011;21:264-70.
- Meyer JJ, Foster RD, Lev-Cohain N, et al. Ann Surg Oncol. Epub 2015 May 12.
- Rusthoven KE, Kavanagh BD, Burri SH, et al. J Clin Oncol. 2009;27:1579-84.
- Rule W, Timmerman R, Tong L, et al. Ann Surg Oncol. 2011;18:1081-7.
- Lee MT, Kim JJ, Dinniwell R, et al. J Clin Oncol. 2009;27:1585-91.
- Herfarth KK, Debus J, Wannenmacher M, et al. Front Radiat Ther Oncol. 2004;38:100-5.
- Ahmed KA, Fulp WJ, Berglund AE, et al. Int J Radiat Oncol Biol Phys. 2015;92:837-42.
- Chang DT, Swaminath A, Kozak M, et al. Cancer. 2011;117:4060-9.
- Scorsetti M, Comito T, Tozzi A, et al. J Cancer Res Clin Oncol. 2015;141:543-53.
- Karanicolas PJ, Metrakos P, Chan K, et al. Curr Oncol. 2014;21:e129-36.
- Adam R, Avisar E, Ariche A, et al. Ann Surg Oncol. 2001;8:347-53.
- Ammori JB, Kemeny NE, Fong Y, et al. Ann Surg Oncol. 2013;20:2901-7.
- Kooby DA, Fong Y, Suriawinata A, et al. J Gastrointest Surg. 2003;7:1034-44.
- Tomlinson JS, Jarnagin WR, DeMatteo RP, et al. J Clin Oncol. 2007;25:4575-80.
- Fong Y, Fortner J, Sun RL, et al. Ann Surg. 1999;230:309-18.