Oct 24, 2014
Introduction
Barbara Burtness, MD
Yale University School of Medicine
The role of induction chemotherapy in managinglocally advanced head and neck cancerhas been under exploration—and underdebate—for three decades. The rationalefor increasing exposure to systemic therapyhas been expounded, and hints have beenextrapolated from meta-analysis, or fromtrials comparing various induction regimenswith each other, but not with definitive therapynot preceded by induction chemotherapy.
As Dr. Anthony J. Cmelak and Dr. Jill Gilbertoutline in their debate, there had been reasonto expect a modest benefit from induction,but trial designs were flawed. Recently,two randomized trials of chemoradiationpreceded or not preceded by docetaxel,cisplatin, and 5-fluorouracil chemotherapyhave been reported.1,2 Each trial enrolled farmore patients with human papillomavirus(HPV)-associated disease than expected,resulting in low event rates and poor statisticalpower; each study also closed beforeattaining its projected sample size. One trialdid not use cisplatin during chemoradiation,so it may either be seen as a test of cisplatinor of induction chemotherapy; the otherused a novel docetaxel/radiation regimen forpatients whose disease did not respond todocetaxel-containing induction. Both trialswere negative. Although low sample size andevent rate compromised the power of eachstudy, the data did not tend to support useof induction. However, new trials continueto generate intriguing results; these trialsemploy biomarkers or HPV status to applyinduction chemotherapy to more treatmentsensitivecancers, exploit induction chemotherapyas a dynamic indicator of treatmentresponsiveness, or capitalize on reducedtumor burden after induction to de-intensifydefinitive therapy.
Dr. Burtness is the Clinical Research ProgramLeader of the Head and Neck Cancers Program,Co-Director of the Developmental TherapeuticsResearch Program, and Professor of Medicine atYale University School of Medicine. She has beenan ASCO member since 1995 and is a Track Leaderof the Scientific Program Committee.
References
1. Haddad R, O’Neill A, Rabinowits G, et al. Lancet Oncol. 2013;14:257-64.
2. Cohen EE, Karrison TG, Kocherginsky M, et al. J Clin Oncol. 2014;32:2735-43.
 |
|
| (Click here to view table) |
The Answer IsYes: Induction Chemotherapy Can Often ImproveTreatment Outcomes
Anthony J. Cmelak, MD
Vanderbilt-Ingram Cancer Center
Induction chemotherapy (ICT) may theoreticallyenhance treatment outcomesin patients with locally advanced headand neck cancer through several mechanisms:by decreasing tumor volume,and therefore the fraction of hypoxiccells that contribute to radioresistance;by bringing about chemosensitization,particularly in combination with platinsand taxanes; and by eliminating clinicallyoccult micrometastatic disease.
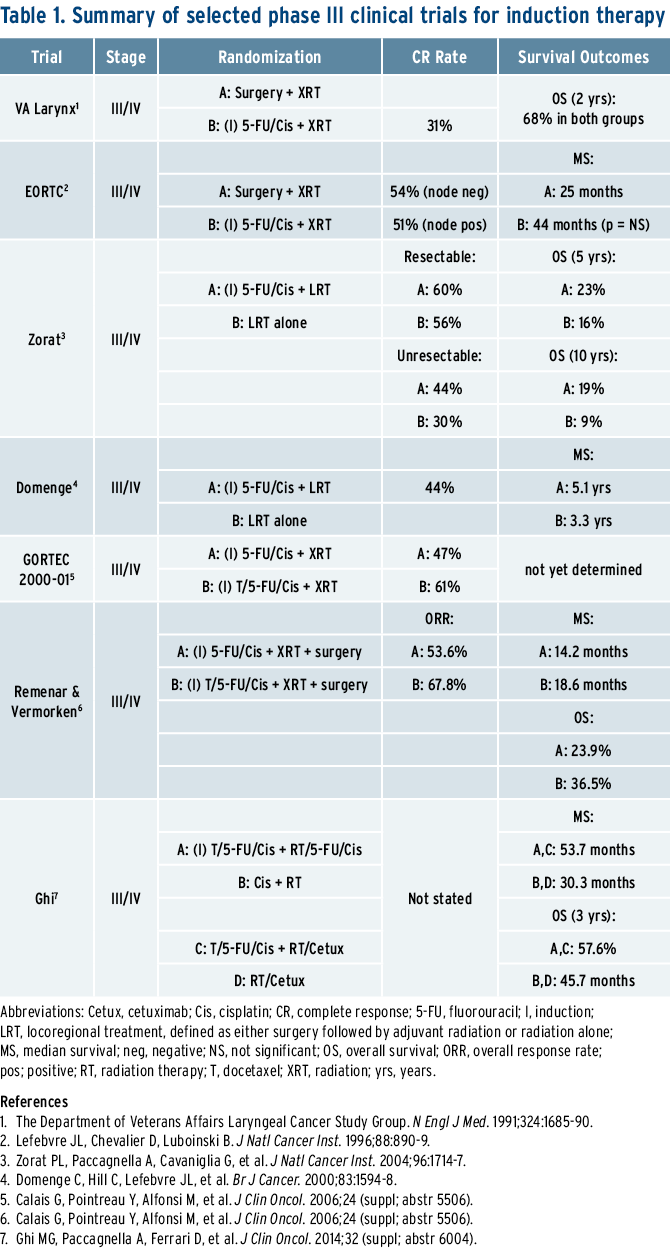
Interest in ICT developed in theearly 1980s after Kish reported highresponse rates to chemotherapy inpreviously untreated patients withlocally advanced head and neck cancerwho received ICT; cisplatin and5-fluorouracil (PF) achieved an 89%overall response rate in inoperablepatients.1 After Kish’s trial, other trialsshowed that ICT response has prognosticvalue in predicting subsequentresponse to radiotherapy.2 These trialsled to investigations utilizing ICT inthe setting of organ preservation. Forexample, the Department of VeteransAffairs (VA) Laryngeal Cancer StudyGroup and the European Organisationfor Research and Treatment of Cancer(EORTC) published sentinel randomizedphase III trials comparing ICT followedby radiation (RT) to laryngectomy andadjuvant radiation.3,4 In both trials,ICT using PF followed by RT resultedin long-term survival comparable tothose undergoing upfront laryngectomyand postoperative RT. Anotherstudy by Zorat et al. tested the role forICT before definitive treatment, eitherRT or surgery, in locally advanced disease.5 As in the VA and EORTC trials,a significant decrease in the incidenceof distant metastases was noted inthe cohort who received induction PF.ICT did not increase survival in thepatients with resectable disease. However,among a subgroup of patientsineligible for surgery, ICT resulted inan increase in complete remission rate(CRR) following all treatment (44% vs.30%, respectively), as well as in five and10-year survival (23% and 19% vs.16% and 9%, respectively). Similarly, astudy by Domenge reported improvedsurvival from 3.8 to 5.1 years in 318patients with oropharyngealcancerrandomly assigned to ICT or no induction.6
The findings in the preceding paragraphwere validated in the mostrecent MACH-HN meta-analysis, whichshowed a small improvement in survivalfor ICT with regimens containing PFcombinations (2.4% at five years, HR0.8; 95% CI [0.77-1]).7 Furthermore,randomly assigned trials using tripletICT with the addition of a taxane havefurther improved survival over PF. Forexample, GORTEC, a phase III trial of220 patients with locally advancedlaryngeal and hypopharyngeal cancer,randomly assigned patients to eitherICT with PF or TPF (docetaxel/cisplatin/5-FU).8 Overall response rate was 83%in the TPF arm (61% CRR) versus 61%(47% CRR) in the PF arm. The threeyearlarynx preservation rate was 73%with TPF versus 63% for PF. The TPFchemotherapy compliance rate of 81%was higher than the PF compliance rateof 67% due to the lower dose of 5-FUneeded. Similar results were seen inother phase III trials evaluating TPF.9,10
Any discussion of ICT in the treatmentof locally advanced head and neck cancermust mention the landmark study,TAX 324. TAX 324 compared inductionwith TPF versus PF, both followed byconcurrent weekly carboplatin with RT. TPF-treated patients had a mediansurvival of 70.6 months versus 30.1months, respectively.10,11 Critics of TAX324 state the cumulative toxicity ofTPF can potentially preclude the useof cisplatin during radiation, hencethe use of carboplatin given with RT.However, a recent four-arm randomizedtrial in 421 patients presented atthe 2014 ASCO Annual Meeting suggeststhat TPF-ICT versus PF-ICT doesnot hinder the subsequent delivery ofcisplatin/5-FU with radiation, and, infact, the addition of a taxane improvedthe median progression free survival(PFS; 37 vs. 21 months) and possiblythe overall survival (OS; 53.4 vs. 41months; HR 1.02, 95% CI [0.70-1.671]).12
On a more investigative note, patientson ECOG 1308 with HPV-related oropharyngealcancers were given threecycles of ICT cisplatin, paclitaxel, andweekly cetuximab. Patients obtaining acomplete endoscopic tumor responsereceived reduced-dose intensitymodulatedradiation therapy at 54Gy with weekly cetuximab. Results,presented at the 2014 ASCO AnnualMeeting, showed a 96% PFS and OS attwo years in complete responders toICT who had nonbulky tumors (T1-T3),minimal smoking history (less than 10packs per year), and no contralateralnodal disease. Long-term toxicitieswere minimal.13 This de-intensificationstrategy will be tested in future studieswith the hope of minimizing toxicitywhile maintaining high cure rates.
In summary, ICT has a legitimate role inthe setting of organ preservation andunresectable tumors. It should also bea consideration for patients with a highrisk of developing metastatic disease,such as those with advanced nodalstage, particularly in regimens that donot hinder the subsequent delivery ofchemoradiation. Novel ICT regimenswill continue to be studied as a paradigmto minimize toxicities in patientswho are HPV+.
Dr. Cmelak is a radiation oncologist,Professor of Radiation Oncology, and theSenior Medical Director in the Departmentof Radiation Oncology at the Vanderbilt-Ingram Cancer Center at Franklin. He hasbeen an ASCO member since 1999 andis currently a Track Leader in the CancerEducation Committee.
References
1. Kish J, Drelichman A, Jacobs J, et al.Cancer Treat Rep. 1982;66:471-4.2. Ensley JF, Jacobs JR, Weaver A, et al.Cancer. 1984;54: 811-4.
3. The Department of Veterans Affairs LaryngealCancer Study Group. N Engl J Med.1991;324:1685-90.
4. Lefebvre JL, Chevalier D, Luboinski B. et al. JNatl Cancer Inst. 1996;88:890-9.
5. Zorat PL, Paccagnella A, Cavaniglia G, etal. J Natl Cancer Inst. 2004;96:1714-7.
6. Domenge C, Hill C, Lefebvre JL, et al. Br JCancer. 2000;83:1594-8.
7. Pignon JP, le Maitre A, Maillard E, et al.Radiother Oncol. 2009;92:4–14.
8. Calais G, Pointreau Y, Alfonsi M, et al. JClin Oncol. 2006;24 (suppl; abstr 5506).
9. Remenar E, Van Herpen C, Lluch JG, et al.J Clin Oncol; 2006;24 (suppl; abstr 5516).
10. Posner MR, Hershock DM, Blajman CR, etal. N Engl J Med. 2007;357:1705-15.
11. Lorch JH, Goloubeva O, Haddad RI, et al.Lancet Oncol. 2011;12:153-9.
12. Ghi MG, Paccagnella A, Ferrari D, et al. JClin Oncol. 2014;32 (suppl; abstr 6004).
13. Cmelak A, Li S, Marur S, et al. J Clin Oncol.2014;32 (suppl: abstr 6006).
The Answer Is No: This Type of Induction Chemotherapy/Regimen Intensity Might Not Be Necessary
Jill Gilbert, MD
Vanderbilt-Ingram Cancer Center
TAX 324 has been hailed as a paradigm-changing study for the treatmentof locally advanced head and neckcancer. TAX 324 was well-designed and evaluated two different induction regimensfollowed by concurrent chemoradiotherapy(CCR).1,2 However, althoughTAX 324 provides us with very importantclinical information, this studywas not designed to ask the questionof whether induction chemotherapy(ICT) is needed. The study, built on theassumption that ICT is important priorto CCR, was designed to further refineand determine the best ICT regimen.Although a subset analysis of TAX 324demonstrated an impressive rate ofclinical benefit among patients withHPV+ oropharyngeal cancer, this findingshould be taken with a grain of saltsince in a “good-risk” population, thistype of chemotherapy/regimen intensitymay not even be necessary.
The TAX 324 subset analysis demonstratedan 87%, three-year overallsurvival (OS) rate for the HPV+ group.But this rate is not significantly differentfrom the 82%, three-year OSrate seen in the RTOG 0129 concurrentchemoradiotherapy trial.2,3 Moreover,it is recognized that tobacco use hasa significant negative impact on clinicaloutcomes in patients with HPV+oropharyngeal cancer, and tobaccouse was not defined in the HPV+ TAX324 subset analysis.4 It is also unclearwhether this level of intensive treatmentis warranted in a nonsmoking,“good-risk” HPV population. That is,given the “normalized” life expectancyof this group of patients, it is worthasking how the late effects of this moreintensive approach hinder the qualityof survivorship. While the long-termfollow-up report of TAX 324 used thepresence of tracheostomy and gastricfeeding tubes as surrogate markersof late toxicity, these markers do notindicate the significant breadth of lateeffects and functional deficits that canaffect this population and which arecritical to report on in a curable population.3
Several randomized studies have asked the question: does ICT improve clinicaloutcomes compared to CCR alone?Unfortunately, either the studies havedemonstrated negative results, wereterminated early for poor accrual, orhave had methodological concerns.However, a trial reported by Ghi etal. at the 2007 ASCO Annual Meetingmay help to answer this question. Thephase III, randomized trial comparedICT followed by CCR to CCR alone.5The study’s primary endpoint wasthree-year overall survival (OS) forthe induction versus non-inductioncohorts. Eligible primary sites includedoropharynx, oral cavity, and hypopharynx.The induction regimen consistedof TPF and the concurrent arms werefurther randomized to a concurrentCCR regimen of cisplatin and 5-FU versusweekly cetuximab with RT. For thestudy population as a whole, regardlessof primary site, both PFS and OS weresignificantly greater in the inductionarms. However, when primary tumorsubsites were analyzed, this statisticalsignificance was lost in the oropharyngealsubset, but remained significantfor the non-oropharyngeal primarysites (oral cavity and hypopharynx).This distinction between the oropharyngealsubset and the non-oropharyngealprimary sites is important; whileHPV status was not reported, one canassume that the majority of oropharyngealtumors are HPV+, suggestingthat the subgroup that was HPV+ maynot need the extra intensity of inductionchemotherapy, while the subsitesthat were non-HPV+ may benefit fromthis approach and, possibly, furtherescalation of intensity. However, itshould be remembered that tobaccouse is associated with worse outcomesin patients who are HPV+, and we awaitthe published manuscript of the Ghi etal. study to see if the authors reportoutcomes in a further subset of thepopulation with HPV+ tumors. Otherwise,further studies will need to lookat this subset of patients to see if theybenefit from an induction approach.To participate in this discussion, visit ASCOconnection.org and select“Magazine,” then “Current Controversies in Oncology,” then title.In conclusion, what this means forinduction chemotherapy is the following:first, induction should notbe applied to “all comers,” and mostpatients who are HPV+ can likelyforego induction chemotherapy. Second,while a subset of patients whoare HPV+ might ultimately benefitfrom induction chemotherapy—forexample, smokers—more studies areneeded to better answer this questionbefore applying this toxic therapy tothat population. And third, inductionchemotherapy likely has the greatestclinical benefit in patients who are non-HPV+, but we await published resultsfrom recently reported trials.
Dr. Gilbert is a medical oncologist, Associate Professor of Medicine (Hematology/Oncology), and the Director of the Hematology/Oncology Fellowship Program at Vanderbilt-Ingram Cancer Center. She hasbeen an ASCO member since 2000. Shecurrently serves as an Associate Editor onthe ASCO University Editorial Board andis Subcommittee Chair of the ProfessionalDevelopment Committee’s Oncology Training Program.
References
1. Posner MR, Hershock DM, Blajman CR, etal. N Engl J Med. 2007;357:1705-15.
2. Lorch JH, Goloubeva O, Haddad RI, et al.Lancet Oncol. 2011;12:153-9.
3. Posner MR, Lorch JH, Goloubeva O, et al.Ann Oncol. 2011;22:1071-77.
4. Ang KK, Harris J, Wheeler R, et al. N Eng JMed. 2010;363:24-35.
5. Ghi MG, Paccagnella A, Ferrari D, et al. JClin Oncol. 2014;32 (suppl; abstr 6004).
